ISI 18-1e Determination of Hydroxyethyl Content in Starch
| 1. Scope | The method is applicable to hydroxyethylated starch |
LT 20.06.1966 Rev. 17.03.1998 |
| 2. Principle |
The hydroxyethyl ether is cleaved in boiling HJ-solution. The ethylene
is distilled and titrated.
|
|
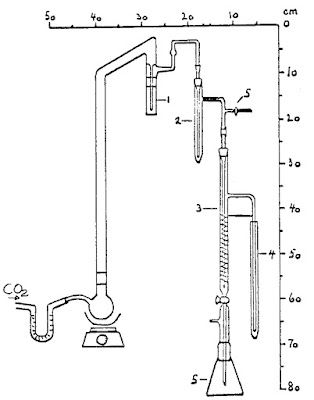
| 3. Apparatus |
3.1 Analytical balance weighing to the nearest 0.1 mg.
3.2 Oil bath, thermostatic 3.3 Distilling apparatus with five collection flasks
|
|
| 4. Reagents |
4.1 Iodine hydrogen solution (HJ) for alkoxide determination; density
1.70; boiling point 126 -127 oC
4.2 Alcoholic silver nitrate solution: 15.0 g AgNO3 is dissolved in 28 ml distilled water. The solution is poured into 422 ml 95% ethanol. A few drops of concentrated nitric acid are added. 10.00 ml of the solution is titrated with (4.3) and with (4.4) as indicator (according to Vogel). Titre = A. Store solution in brown flask. 4.3 Ammonium thiocyanide 0.05N NH4SCN. Normality = N2 4.4 Ferric sulphate indicator: Nitric acid is added to a saturated solution until the brownish colour disappears. 4.5 Bromine-acetic acid solution: 600 ml conc. acetic acid is saturated with dry KBr (approx. 10 g). 2 ml bromine is added. 15.00 ml is titrated with (4.6). Titre = B. Keep dark and cool in brown tight flask. The solution is stable a couple of days after preparation. 4.6 Na-thiosulphate solution 0.05N Na2S2O3. Normality = N1 4.7 Potassium iodine solution, 10%: 10 g KJ ad 100 ml with distilled water. 4.8 Starch indicator, 1%: 1 g ad 100 ml with distilled water. 4.9 Cadmium sulphate, 5%: 5 g ad 100 ml with distilled water. 4.10 Red phosphorous, amorphous powder. 4.11 Carbon dioxide, dried gas
|
|
| 5. Procedure | Weigh approximate 1 g (g) water free sample to the nearest mg into the distillation flask and add 40 ml (4.1). | |
|
Prepare distillation apparatus (3.3) and fill the flasks:
Washing flask no.1: 0.5 g (4.10) and add (4.9) to 3 cm above inlet tube. Collection flask no. 2: 10.00 ml (4.2) Collection flask no. 3. 15.00 ml (4.5) Collection flask no. 4: 15.00 ml (4.7) Flask no. 5: 10 ml (4.7) and 150 ml distilled water |
||
| Flask and cooler is assembled immediately. Carbon dioxide (4.11) is added | one bobble/s | |
|
Cleavage |
The distillation flask is heated on oil bath kept at 140 - 145 oC for one hour and heating is stopped. | |
| 1. Heat collector no 2 to 40 - 50 oC | ||
| 2. Open valve no 5 slowly and close CO2 supply | ||
| 3. Empty collector no 3 and 4 into a 500 ml flask. | ||
|
Titrate C2H4 |
4. Flush with distilled water and titrate immediately with (4.6) and 2 ml (4.8) as indicator. Titre = a. | |
| 5. Empty collector 2 into a 500 ml flask with 150 ml distilled water and flush. Heat to boiling and cool. | ||
|
Titrate C2H5J |
6. Titrate with (4.3) and 3-5 ml (4.4) as indicator. Titre = b. | |
| 7. Run a blank with 1 g native starch. | Blank on potato starch is approx. 0.2% C2H4O | |
| 6. Calculation |
Calculate ethylene-oxide content of sample dry matter by averaging
results of two samples with two decimals.
C2H4O % = x + y - blank, where x = 2.203 (A-a) N1 / g = C2H4O % distilled as C2H4 y = 4.405 (B-b) N2 / g = C2H4O % distilled as C2H5J
|
Molecular weight of C2H4O = M = 44.05 |
| 7. Reference |
H. J. Lortz; Anal. Chem. 28, 892 (1956)
|
|
| 8. Diagram | ||

Comments
Post a Comment