ISI 20-1e Determination of Sulphur Dioxide in Starch
| 1. Scope |
The method is applicable to native and modified starch.
|
LT 13 Sep 1999 |
| 2. Principle |
Sulphur dioxide is released by boiling with acid. The gas is collected
in hydrogen peroxide and oxidised to sulphuric acid and tirated with
alkali.
|
|
| 3. Apparatus | 3.1 Analytical balance weighing to the nearest 1 g. | |
|
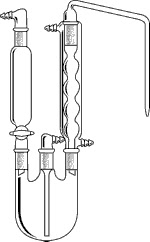
3.2 Monier-Williams Sulphites Distillation Apparatus consisting of a three neck 1000ml boiling flask with a sloping
Allihn reflux condenser, a right angle inlet adapter with a bubbler tube
(scrubber), a 60-ml dropping funnel and 500 ml conical flask (collector
/ receiver) 3.3 Electrical heater
|
||
| 4. Reagents |
4.1 Sodium carbonate solution: Dissolve approx. 15 g of
Na2CO3 or 40 g of
Na2CO3.10H2O in distilled water and
dilute to 100 ml. 4.2 Hydrogen peroxide, 2%: Dilute 10 ml of neutral 30% hydrogen peroxide (H2O2) with distilled water to 100 ml. 4.3 Hydrochloric acid, concentrated (HCl) 4.4 NaOH 0.1N 4.5 Bromophenol blue 0.1% indicator (C19H10Br4O5S): Dissolve 0.1 g of bromophenol blue in 1.5 ml NaOH 0.1N (4.4) and 20 ml alcohol and dilute to 100 ml with distilled water.
|
|
| 5. Procedure | Carbon dioxide is passed through the scrubber with sodium carbonate solution (4.1) and into the gas-inlet tube of the boiling flask. | The scrubber removes chlorine |
| Place 20 ml hydrogen peroxide solution (4.2) in the receiving flask | ||
| Add through the dropping funnel 300 ml distilled water and 20 ml concentrated hydrochloric acid (4.3) | ||
| Assemble and start cooling. Boil for 10 min. in a current of carbon dioxide | ||
| Weigh 100 g of sample to the nearest g (w) and disperse in 300 ml of fresh boiled distilled water. Transfer the slurry to the boiling flask via the dropping funnel | Adjust dropping rate and gas flow to prevent drawback of hydrogen peroxide or air | |
| Boil gently for one hour with a slow current of carbon dioxide. | ||
|
Titration |
Add immediately indicator (4.5) and titrate with 0.1N NaOH | |
|
Blank |
Carry out a blank test with water and reagents
|
|
| 6. Calculation | Calculate sulphur dioxide content of sample by averaging results of two samples with two significant digits: | |
|
Sulphur dioxide % = ((s-b) * 0.0032 *100) / w where
s = ml 0.1N sodium hydroxide for sample Correct eventually to sulphur dioxide on sample dry matter.
|
||
| 7. Notes |
Additional gravimetric determination by precipitation with barium
chloride after titration.
|
|
| 8. Reference |
JECFA
|
|
| 9. Monier-Williams Sulphites Distillation Apparatus |
|
A slanting Allihn condenser is preferred in stead of the one shown. Connect the gas inlet tube to a scrubber and carbon dioxide source. Connect the outlet to a receiving flask. |

Comments
Post a Comment